Symptom Monitoring Increases Survival
Results of a randomized trial of patient-reported symptom monitoring during cancer treatment is shown to increase overall survival over standard care.
In a plenary session of the ASCO Annual Meeting, Ethan Basch of the University of North Carolina reported the results of a decade-long study measuring the impact of monitoring symptoms on Quality of Life (QOL) and overall survival. Presentations at plenary sessions at ASCO are reserved for studies “having practice-changing findings of the highest scientific merit”. The presentation by Ethan Basch fits that category and, if his findings are successfully duplicated in a large scale, national trial, could change the way clinicians manage their patients – to the benefit of all cancer patients.
The study’s rationale was that cancer patients do not adequately report the frequency and severity of their symptoms at their regular office visits due to their, or the clinicians’, forgetting the details covered and lack of time during the office visits. Likewise, the patient is often reluctant to contact their physician, or even unable to, with regard to their symptoms until they become severe enough to land the patient in emergency care. But, in the computer age, where people constantly use social media to post frivolous information about themselves, one would think that electronic communication could be used to improve the care of cancer patients.
Dr. Basch’s investigative team captured patients’ symptoms during the interim period between office visits via a simple form filled out by the patients when prompted by a weekly email reminder. If the patient’s symptoms are severe or worsening, an email alert is automatically sent to the physician’s nurse. A report is also sent to the doctor, which can be discussed at the regular office visit. Dr. Basch calls this a “proactive approach” that allows clinicians to “pick up on problems and address them before they cause serious complications”. This approach makes sense especially in light of the fact that clinicians fail to pick up half of a patient’s symptoms, according to previous studies cited by Dr. Basch.
Accordingly, Dr. Basch ran a 766-patient trial at Memorial Sloan-Kettering Cancer Center for breast, lung, genitourinary, and gynecological cancer patients receiving chemotherapy. The patients were enrolled in the study from June 2007 to January 2011. They were randomized into two arms, a Control Arm, in which patients received standard-of-care, and an Intervention Arm, in which they self-reported 12 common symptoms, e.g., pain, in the interim period between visits, prompted by email notices. They could also enter the information via terminals located in the doctor’s waiting area prior to their visit. Severe or worsening symptoms triggered an email to the nurse for immediate action as well as a report to the doctor for reference during a follow-up appointment. The Control Arm reflected standard symptom monitoring.
The following are samples of two of the forms, one for pain and the second for dyspnea (shortness of breath) that a patient would enter an option for.
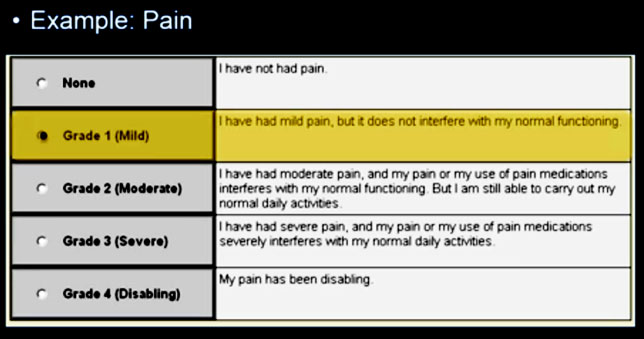
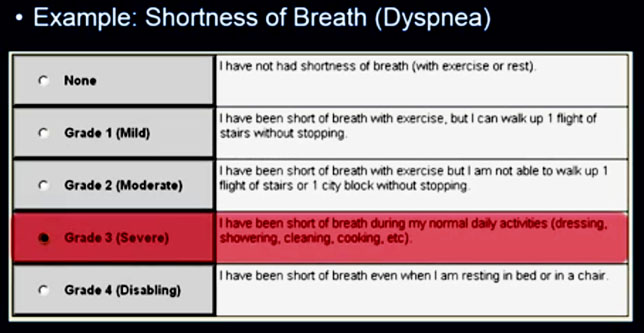
The Grade 3 entry for dyspnea initiates an email alert to the nurse (see below), allowing her to take action between visits based on the increased severity of this symptom.
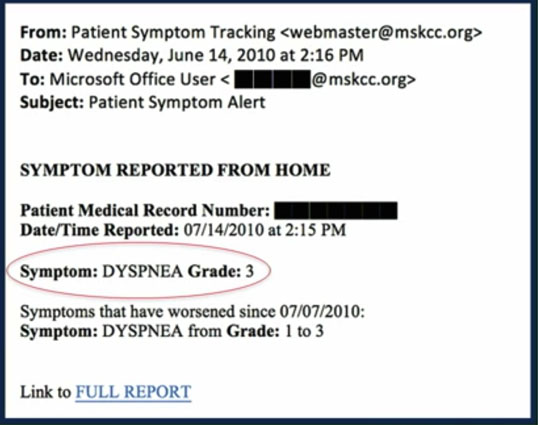
In addition, a report is generated and emailed to the patient’s physician allowing her to review the patient’s profile with him at the time of the next visit.
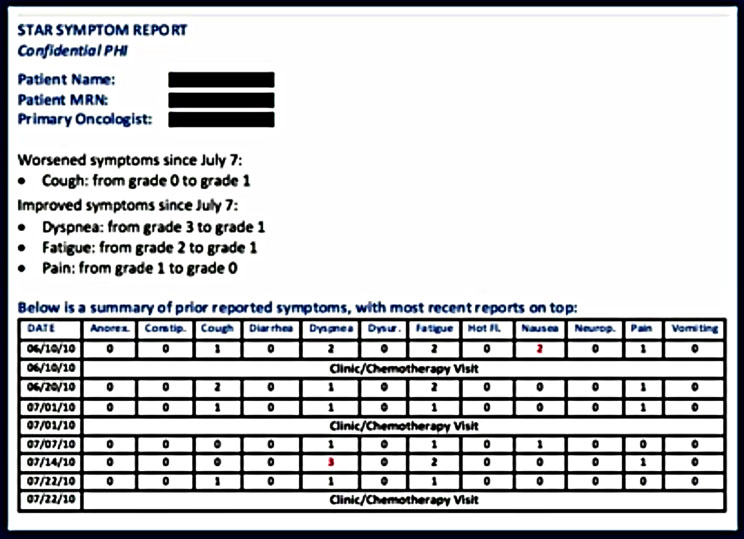
The patients reported 73% of the time after email prompting. Prior computer experience was not a factor in the frequency of response. The nurses who received the email alert took action 77% of the time by: counseling the patient, adding medications and/or modifying chemotherapy doses, referring patients to the ER or to radiology. For the Intervention Arm, 7% fewer patients reported to the ER than those in the standard of care arm.
Results
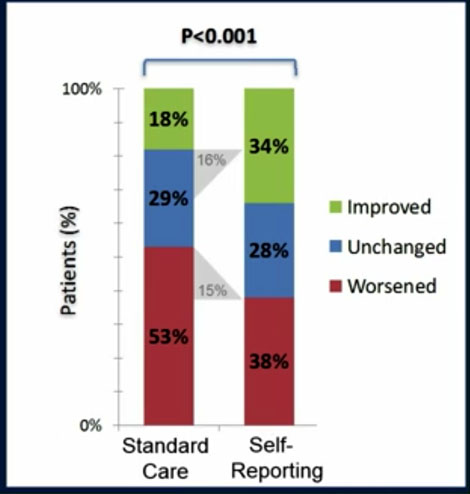
The trial’s objective was to track two important patient indicators: Quality of Life (QOL) and overall survival. QOL was reported in 2016 in the J Clin Oncol 2016;34:557-565. Compared to the standard of care arm, patients in the Intervention Arm reported a 31% increase in benefits in QOL, with 16% of the self-reporting patients experiencing improved QOL and 15% experiencing less worsening of QOL than standard of care patients.
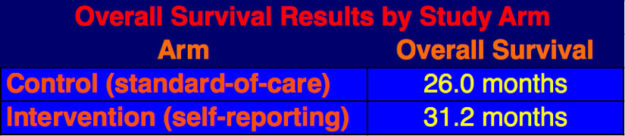
The survival analysis was done in June 2016 after two-third of the patients had died. The data showed that patients on the self-reporting arm had a five-month increase in median overall survival over the standard-of-care arm patients, 31.2 mos. versus 26.0 mos.
Dr. Basch posited three reasons why overall survival improved in self-reporting patients:
- Proactive monitoring prompts clinicians to intervene early, before symptoms worsen and cause serious downstream problems.
- Symptom control enables patients to stay more functional, which keeps them mobile – this is associated with better survival – as reported in previous studies.
- Symptom monitoring improves control of chemotherapy side effects, enabling higher intensity and longer-lasting duration of treatment, in this study, an increase of two months of treatment (8.2 mos. versus 6.3 mos.).
Limitations of the Study
This was a single-center study, although its scope being expanded in a new, national study. Secondly, the technology has improved since 2007 making it easier to use and integrating the reports with the physicians’ electronic records.
Dr. Basch’s conclusions were that:
- Systematic symptom monitoring with web-based patient self-reporting improves clinical outcomes including Quality of Life and overall survival.
- This approach should be considered for inclusion as a part of standard system management as a component of high-quality cancer care.
- Future efforts should focus on implementation strategies for integrating patient self-reporting into electronic health records systems and into the workflow of oncology practice.
A multi-center trial to validate the results of this single-center study is being planned.
