
From October 2014 through June 2017, Bristol-Myers Squibb sponsored a Stage III trial, called CheckMate 214 (NCT02231749 on clinicaltrials.gov), testing the combination of therapies nivolumab (Opdivo) and ipilimumab (Yervoy) against sunitnib (Sutent) in treatment-naïve (first line), clear cell metastatic kidney cancer patients. Based on the results of this trial, the nivolumab (nivo)-ipilimumab (ipi) combination won FDA approval on April 16, 2018, for the treatment of intermediate or poor-risk kidney cancer patients. It was not approved for favorable-risk patients, who fared better on sunitinib than on the nivo/ipi combination. The following was mostly taken from a paper in the NEJM, published April 5, 2018, written by Robert Motzer et al (he is from Memorial Sloan Kettering Cancer Center).
The primary endpoints of the trial were overall survival, objective response rate, and progression-free survival in patients with intermediate and poor prognostic risk, although some patients with favorable risk were included in the trial.
Sunitinib is a tyrosine kinase inhibitor (TKI) of the vascular endothelial growth factor (VEGF) and was considered the standard therapy for the treatment of metastatic clear cell RCC (ccRCC) during the targeted therapy era, from January 2016 through November 2015 when nivolumab was approved for kidney cancer treatment. Nivolumab is a monoclonal antibody that is what’s called a checkpoint inhibitor of the PD-1 receptor on the surface of T-cells, which puts a check on the ability of the immune system to fight the cancer. Ipilimumab is also a monoclonal antibody that targets CTLA-4, a protein that downregulates the immune system.
About three-quarters of metastatic TCC patients have intermediate or poor-risk factors, the rest having a favorable risk factor. See https://www.ackc.org/international-metastatic-rcc-database-consortium-imdc-risk-score/ for risk factor prognostication for survival from metastatic kidney cancer. The nivo/ipi combination has had favorable results in other cancer types and has been approved for the treatment of melanoma.
Around 70% of the metastases in the patient population appeared in the lung with about 20% each in the bone and/or liver.
Efficacy for Intermediate and Poor Risk Patients
The following tables show the efficacy results for intermediate and poor prognoses patients only.
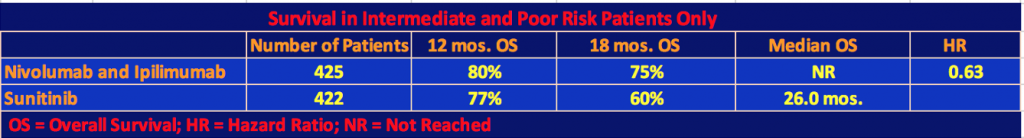
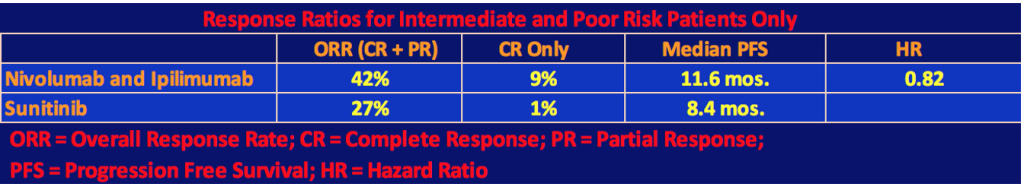
The superiority of the combination therapy of nivolumab plus ipilimumab over sunitinib in intermediate and poor-risk patients who were previously untreated was confirmed by the statistical significance of these results. As the second table shows, nivo/ipi had a longer progression-free survival than sunitinib, however, this difference was not statistically significant as the p value threshold of .009 was not reached. In the two-year follow-up for data analysis, 9% or 40 patients of the nivo/ipi cohort had a complete response. We are now used to seeing long-term responders under checkpoint inhibitor therapy. It will be interesting to see in a future follow-up report on the trial, how many patients will still be responders.
In one final note on efficacy, the investigators plotted the hazard ratio for death for various patient characteristics. Almost all favored nivo/ipi patients except age. For patients under 65 years old, the HR was 0.53, meaning that there was a 47% higher possibility of survival for nivo/ipi patients as compared to sunitinib patients. For patients aged 65-75 years old, the HR was 0.86, still favoring the immunotherapy treatment. But for patients 75 years old and over, the HR was 0.97, only a 3% advantage. Do these statistics mean that the older you are, the less likely you are to respond to immunotherapy?
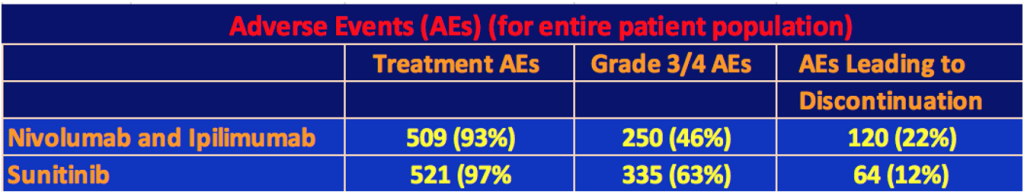
Adverse Events for Total Population
The following table shows the Adverse Events for each therapy. Almost every patient experienced at least one Adverse Event for either therapy, but the Grade 3 or 4 events favored the nivo/ipi combination although there were almost twice as many discontinuations of therapy among the nivo/ipi patients than there were among the sunitinib patients.
The highest specific Grade 3/4 event for the nivo/ipi cohort was increased lipase levels in 10%, which, if serious enough, can cause pancreatitis, kidney failure, cirrhosis of the liver, or bowel problems. 4% of the nivo/ipi patients had Grade 3/4 fatigue or diarrhea. Until this trial, the last use of ipilimumab in kidney cancer was a 2007 study by James Yang of the NCI. In this trial, in a 40-patient cohort, 17 of them had enteritis (inflammation of the small intestine). Three had a perforation of the colon, and two died from the perforation. This seems to have put a damper on the use of ipilimumab in kidney cancer. In this trial, the clinicians must have learned how to deal with these issues to keep the number of gastrointestinal events under control. There were eight treat-related deaths in the nivo/ipi cohort versus four for sunitinib. Of the eight, one was for lower gastrointestinal hemorrhaging. Three of four of the sunitinib treatment-related deaths were cardiac-related.
Efficacy for Total Population: Favorable, Intermediate, and Poor Risk Patients
The following tables include the Favorable Risk patients (125 patients on nivo/ipi and 124 patients on sunitinib) as well as Intermediate and Poor Risks.
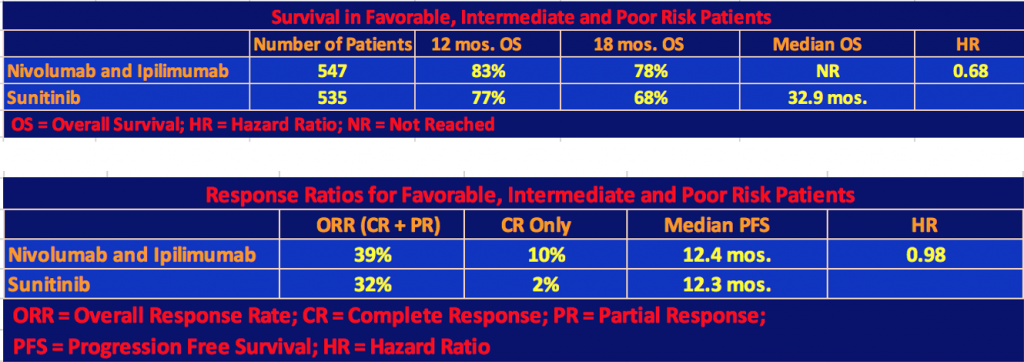
The nivo/ipi cohort still had a significant survival advantage with a hazard ratio for death of 0.68 and a p-value < 0.001. However, the response rate and median progression-free survival differences were not significant since, as we mentioned, the nivo/ipi Favorable Risk cohort did not do as well as the sunitinib one so the differences with respect to the total patient population narrowed.
Finally, the investigators gave the exploratory analysis data for favorable-risk patients.
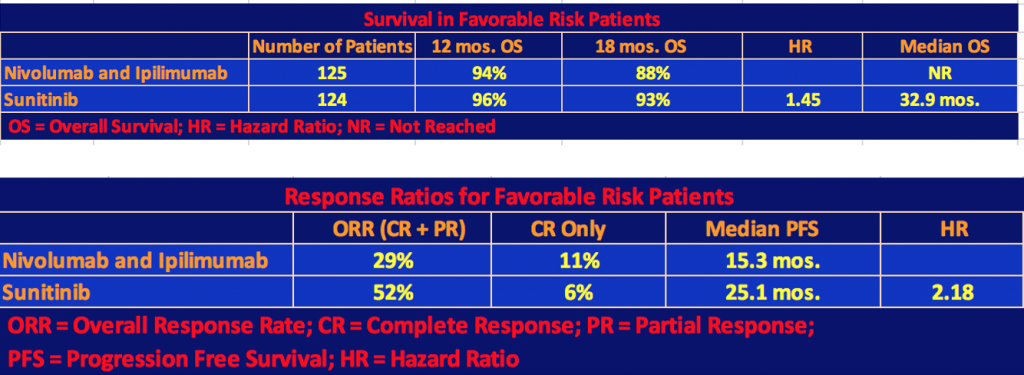
Note that in the first table, the hazard ratio for death, 1.45, favored sunitinib, and in the second table, the hazard ratio for disease progression, 2.18, favored sunitinib as well. But the results were mixed, especially with respect to complete responses and long-term survival of the nivolumab + ipilimumab patients. Quoting Dr. Motzer’s comment, “These results in favorable-risk patients should be interpreted with caution because of the exploratory nature of the analysis, the small subgroup sample, and the immaturity of survival data”.
Discussion
This trial showed that the combination therapy of nivolumab and ipilimumab for treatment-naïve, intermediate or poor-risk patients was clearly superior to the sunitinib monotherapy, which is why it was approved by the FDA. We didn’t cover the biomarker PD-L1, which showed better results for patients with 1% or greater PD-L1 expression because it hasn’t yet been found to be predictive of response or survival for nivolumab treatment. The nivo/ipi cohort also showed a higher quality of life than the sunitinib cohort.
Dr. Motzer also mentioned in his discussion that the standard regimen of treatment by sunitnib of four weeks on, two weeks off was used in his trial. Some patients find this too toxic and are on a two-week on, one-week off schedule. That latter schedule, if it had been used in this trial could have affected efficacy and/or adverse events. Even so, twice as many nivo/ipi patients discontinued therapy than sunitinib patients. Treatment with prednisone, a steroid, was used to reduce the toxicity of the immunotherapy with respect to gastrointestinal issues.
One comment on the design of the trial. Bristol-Myers Squibb designed the trial to have only two arms, the nivolumab plus ipilimumab combination versus sunitinib. They should have also had an arm with nivolumab as a monotherapy. If they had, they could have analyzed the differences in efficacy and toxicity among the three therapies, and future potential patients and clinicians would have had a better understanding of the trade-offs among the therapies when deciding which one to take, or prescribe.
A final editorial comment. The sex breakdown in the nivo/ipi cohort in this trial was 75% male to 25% female, or a three to one ratio. The American Cancer Society projections for 2020 show the male/female breakdown for kidney cancer incidents to be 62% versus 38% or a 1.6 ratio. Even for mortality date, the ACS breakdown is 66% versus 34%, close to two-to-one. It has been shown in other cancer studies that women respond differently to therapy than do men. Kidney cancer trial designers should endeavor to recruit a trial population that more closely matches the metastatic population’s sex ratio, or, at least, stratify the results by gender.

Fuel our fight to conquer kidney cancer! Engage with our blog and social media to help promote cancer awareness and spread the word in support of increased research funding. Join our 11K+ followers on Facebook.
We invite you to comment here!